VIDAS® PTH (1-84)
3rd generation precision
VIDAS® PTH (1-84) is a quantitative test for the determination of the biologically active form of parathyroid hormone in human serum or plasma.
- Accurate monitoring of chronic kidney disease patients
- Direct correlation with International Standard WHO 95/646
- 84-day calibration
Maggiori informazioni
Accurate follow-up of chronic kidney disease - A reduced cost per patient - A user-friendly solution to monitor Bone & Mineral Metabolism
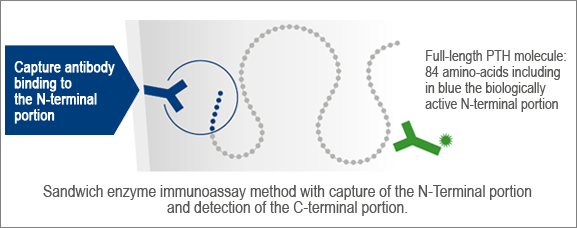
Parathyroid hormone (PTH) is the main hormone involved in calcium and phosphorus homeostasis. PTH measurement is useful in the diagnosis of hyper/hypocalcemia, hyper/hypoparathyroidism, osteoporosis, the monitoring of chronic kidney disease, and peri-operative PTH monitoring in parathyroid surgery. The full-length molecule, PTH (1-84), is rapidly degraded in the bloodstream into fragments that are not biologically active. Usually eliminated by the kidneys, these fragments accumulate in the blood when the kidneys fail to function.
VIDAS® PTH (1-84) is a 3rd generation assay. As such, it recognizes only the active form of PTH, offering clinicians the diagnostic accuracy they need for better patient management.
Accurate follow-up of chronic kidney disease
In chronic kidney disease (CKD) patients, bone and mineral disorders worsen as the disease progresses. International guidelines recommend regular PTH measurement to monitor calcium homeostasis and adapt treatment1. However, the non-active PTH fragments that accumulate in the blood of dialysis patients act as important confounders. This is where greater specificity is essential and clinicians recognize the analytical value of 3rd generation PTH tests over 2nd generation tests2.
With its unique test format, the VIDAS® PTH (1-84) assay is specific for the biologically active form of PTH. The absence of cross-reaction with non-active PTH fragments makes it particularly suitable for accurate follow-up of CKD patients.
VIDAS® PTH (1-84) test format
According to the KDIGO guidelines, the PTH concentration in dialysis patients should be maintained within 2 and 9 times the upper normal limit of the assay1.
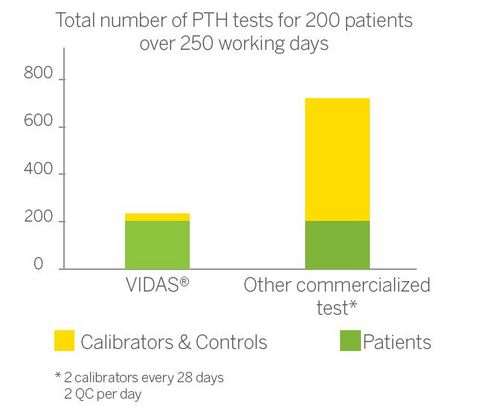
A reduced cost per patient
Make the choice of cost-effectiveness with our VIDAS® PTH (1-84) test:
- 84-day calibration: save money and time!
- On-demand testing adapted to small / medium volumes
- Calibrator and control included in the kit
A user-friendly solution to monitor Bone & Mineral Metabolism
The VIDAS® PTH (1-84) assay is run on the VIDAS® family of instruments. Perform other Bone & Metabolism assays on the same platform to easily monitor chronic kidney disease patients:
References:
- KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59.
- Cavalier E, et al. Ann Endocrinol (Paris). 2015 May;76(2):128-33.
- See package insert.