Quality & Compliance Services
We’re your partner on the path of risk assessment and Quality compliance for better health outcomes
- Comprehensive, personalized Environment Monitoring with LABGUARD®
- Expert Validation Solutions just for you
- Full range of Quality Control monitoring in immunoassays (myQC for VIDAS instrument)
Maggiori informazioni
As we aim to continually improve patient care while managing a higher workload and the need to keep costs down, the regulatory environment is becoming ever-more stringent. Ensuring Quality and Compliance management has never been more important. Working with bioMérieux for your Quality and Compliance management will help give you the confidence of knowing it is all under control. Be prepared for the future.
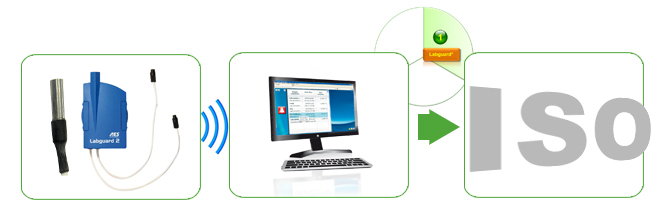
Environmental monitoring
Environment Monitoring is an essential but time-consuming and increasingly complex part of regulatory compliance, quality and laboratory safety. We work with you to create a monitoring program tailored to you lab’s specific needs with an integrated and wireless system for real-time monitoring of temperature and other parameters
- Real-time Environmental Monitoring: LABGUARD® is an innovative, high-quality real-time wireless monitoring system for temperature and physical parameters. This centralized “cockpit” control system is the best solution to secure samples and monitor both storage and transportation conditions. It gives the information needed for you to rapidly implement corrective action – before sample integrity or product performances are impacted by any non-conforming storage or shipping conditions.
- Hardware, software and services
- Wide range of probes, transmitters, data loggers & more
- Effective, smooth operation
- Unique Services: bioMérieux guarantees you a long-term quality support and training granted by our dedicated LABGUARD® teams. We work hard to make sure the LABGUARD® system evolves with your growing needs.
- Meets accreditation standards, improves daily operation: LABGUARD® assures you satisfy laboratory accreditation EN15189 standards. Get peace of mind – knowing LABGUARD® helps you both prove the quality of your procedures, and simplify your audits and accreditations.
Trusted experience and expertise
LABGUARD® is a worldwide solution with over 15 years’ experience. Our Service laboratory (metrology, calibration for temperature) is ISO 17025 accredited.
Validation Solutions
Accreditation requirements include laboratory validation – an increasingly complex and time-consuming process. To help you through the validation maze and save valuable time, we offer personalized step-by-step validation services to help you quickly and correctly validate your bioMérieux instruments. In your lab context, we can help you with:
- Validation documents: We provide the documents to complete your validation process and set-up sustainable documentation streams. This helps you qualify systems, validate reagents before routine use, and verify methods under user conditions.
- Validation tools: Our dedicated software tracks your system validation performance to automate calculation and make validation easier. Conclusion and validation will be based on customer approvals.
- Training your technicians to validate systems: A dedicated bioMérieux expert works with you to start the validation process. Tailored to suit you, the training includes demonstration of appropriate use of instruments, user manuals, method validation software, as well as answers to your pressing compliance and quality questions.

Risk analysis procedure
To meets your needs, bioMérieux has developed guidelines to implement a risk analysis approach to answer ISO 15189 requirements.
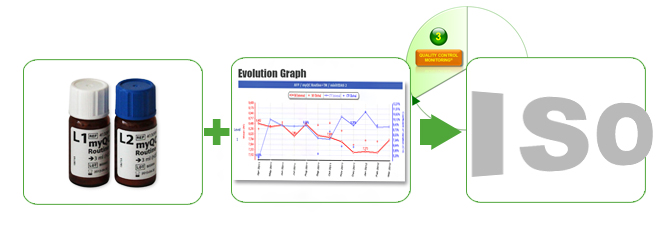
Quality Control Monitoring
To help you be compliant with ISO & CAP standard requirements, bioMérieux Performance Solutions offers a complete full range of services – myQC solutions (Internal Quality Control (IQC)) – for immunoassays (VIDAS® instrument). IQC rapidly and successfully ensures patient results quality through early drift detection and evaluates result bias with analytical performance through peer-to-peer comparison.
- Quality Controls (QC) Material (vials): 3 types of IQC for VIDAS® Instruments.
- QC ROUTINE + for main Immunochemistry parameters (VIDAS® Thyroid, VIDAS® Tumor Markers, VIDAS® Fertility,…)
- QC CARDIAC with critical care parameters (VIDAS® DDimer II, VIDAS® NT Pro BNP, VIDAS® Troponin I, VIDAS® Myoglobin)
- QC SPECIALTY for VIDAS® B.R.A.H.M.S PCT
- Web application for QC peer-to-peer groups sharing: soon available to enable VIDAS® instruments customers (VIDAS®, mini VIDAS® and VIDAS® 3), to compare their internal quality with others lab professionals worldwide. Can connect to instruments through VILINK™ software.
- New services dedicated to accreditation & QC analysis: beyond our basic customer after-care support, you can gain the advantage of new dedicated phone and onsite services when you need them.
myQC for VIDAS®
To allow customers to locally monitor their Vidas performance, have access to peer comparison with the largest based of Immunoassays system worldwide in clinical laboratories.
- Labguard Brochure
- Labguard website
- myQC for VIDAS®